As understood success does not suggest that you have astonishing points. One electron added will fill that shell.
Electronegativity is related with ionization energy and electron affinity.

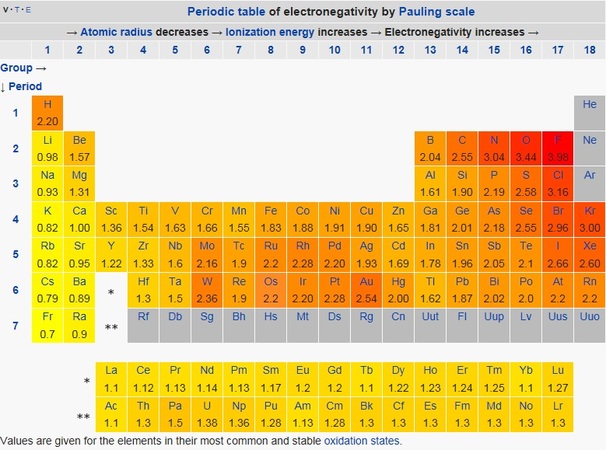
. Of the main group elements fluorine has the highest electronegativity EN 40 and cesium the lowest EN 079. 119 rows Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. Lithium on the other hand has a single valence electron so adding an electron doesnt fill the shell.
Best Answer Copy Fluorine is the most electronegative element. Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Fluorine is the most electronegative element.
Fluorine has the highest electronegativity of all the elements with the largest nuclear charge fluorine has the strongest pull force. Although fluorine is more electronegative than oxygen but the ability of oxygen to stabilise higher. Click to see full answer.
The optimal electron configuration of the 2P orbital contains 6 electrons so since Fluorine is so close to ideal electron configuration the electrons are held very tightly to the nucleus. Fluorine has appropriate values for all of the common scales to ensure it has the highest electronegativity. It has the highest elemental electronegativity by the Allen method at 4193 to hydrogens 2300.
Electronegativity describes the ability of an element to hold onto its electrons and fluorine gathers electrons -. Because the distance term is squared the force drops quickly as the electrons are farther from the nucleus. Values for electronegativity run from 0 to 4.
It can also be used to predict if the resulting molecule will be polar or nonpolar. Is oxygen more electronegative than fluorine. Electronegativity and Bond Type.
A Covalent Bonds Practice Showing top 8 worksheets in the category - Covalent Bonds Practice. Fluorine has an electronegativity of 398 on the Pauling Electronegativity Scale and a valence of 1. Fluorine is actually more electronegative than any other element because it has the greatest nuclear chargeatomic radius ratio its nuclear charge is large relative to its atomic radius meaning that any electrons in a covalent or ionic bond with fluorine will experience a greater pull from the positively charged nucleus of the fluorine atom than that of the other atom in the.
It is an extremely reactive element and a strong oxidising agent. Fluorine is the most electronegative element because the definition of electronegativity makes it so. The higher the associated electronegativity number the more an element or compound attracts electrons towards it.
X is a type of bond that is payable to whoever holds it. A fluorine atom needs one electron to fill its outer electron shell and achieve stability which is why free fluorine exists as the F ion. The electronengativity scales are defined based on experimentally determined properties of the elements.
The most electronegative atom fluorine is assigned a value of 40 and values range down to cesium and francium which are the least electronegative at 07. Fluorine has the highest electronegativity of all the elements the first reason is that fluorine has a very small atomic size and it has 7 Valence Electrons that means that it can attract electrons very easily because size is small and the number of protons are very high that is why fluorine has the highest electronegativity of all the elements. Yes fluorine is more electronegative than hydrogen.
Among the elements it has the highest electron affinity and the third-highest electronegativity behind only oxygen and fluorine. Fluorine has a single empty spot in its valence electron shell. Fluorine is the most electronegative element because it has 5 electrons in its 2P shell.
E formula for a compound. That is again why Flourine is the most electronegative element.
Why Does Fluorine Have Greater Electronegativity Than Iodine Quora

What Is Electronegativity Trends Chart Periodic Table Chemtalk

Fluorine Has The Highest Electronegativity Among The Group On The Pauling Scale But The Electron Youtube

0 Comments